Is Food Coloring Polar or Nonpolar?
Polarity and Solubility

Is food coloring polar or nonpolar – The solubility of a substance, whether it readily dissolves in a solvent or not, is intrinsically linked to its polarity and the polarity of the solvent. This fundamental principle governs how food colorings, with their diverse chemical structures, behave in different environments like water and oil.Polarity describes the distribution of electrical charge within a molecule. Polar molecules possess a positive and a negative end due to an uneven distribution of electrons, while nonpolar molecules have an even distribution of charge.
The polarity of food coloring, often a crucial factor in its solubility and interaction with ingredients, directly impacts its effectiveness. Understanding this is key to achieving vibrant hues, as exemplified by the careful considerations in the application of food coloring, such as detailed in this article on food coloring in cake. Ultimately, the polar or nonpolar nature of the dye dictates its ability to disperse evenly and contribute to the overall aesthetic appeal of the baked good.
“Like dissolves like” is a crucial concept: polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents.
Polar and Nonpolar Regions in Food Coloring Molecules
Food colorings are often complex molecules containing both polar and nonpolar regions. The presence and relative size of these regions determine their overall solubility characteristics. For example, many synthetic food colorings contain benzene rings (nonpolar) and sulfonic acid groups (-SO3H) (polar). The sulfonic acid group is highly polar, making the molecule water-soluble. However, the presence of a significant nonpolar component might reduce its solubility in water and increase its solubility in a less polar solvent like oil, to a certain extent.
The balance between these regions dictates the overall solubility behavior.
An Experiment: Food Coloring Solubility in Water and Oil, Is food coloring polar or nonpolar
To illustrate the relationship between polarity and solubility, a simple experiment can be conducted using a common food coloring, such as red food coloring (often containing Allura Red AC), water, and vegetable oil.The experiment aims to observe the solubility of red food coloring in water (a polar solvent) and vegetable oil (a nonpolar solvent).
- Gather materials: Red food coloring, water, vegetable oil, two clear glasses or jars.
- Add approximately 100ml of water to one glass and 100ml of vegetable oil to the other.
- Add 5-10 drops of red food coloring to each glass.
- Gently swirl each glass to mix the food coloring with the solvent.
- Observe and record the solubility of the food coloring in each solvent over a period of 5-10 minutes. Note any changes in color intensity or the formation of layers.
Observations on Food Coloring Solubility
In the water glass, the red food coloring will likely dissolve readily, resulting in a homogeneous, uniformly colored solution. This is because the polar groups in the Allura Red AC molecule interact favorably with the polar water molecules. In contrast, in the oil glass, the food coloring will likely remain largely undissolved, forming a distinct layer at the interface between the oil and water.
This limited solubility in oil is due to the nonpolar nature of the oil, which has weak interactions with the polar groups of the food coloring molecule. The benzene rings of the dye will interact favorably with the oil, but the sulfonic acid groups will not, leading to a heterogeneous mixture. The extent of the dye’s solubility in the oil will depend on the precise chemical structure of the food coloring and the relative strength of its polar and nonpolar components.
Intermolecular Forces
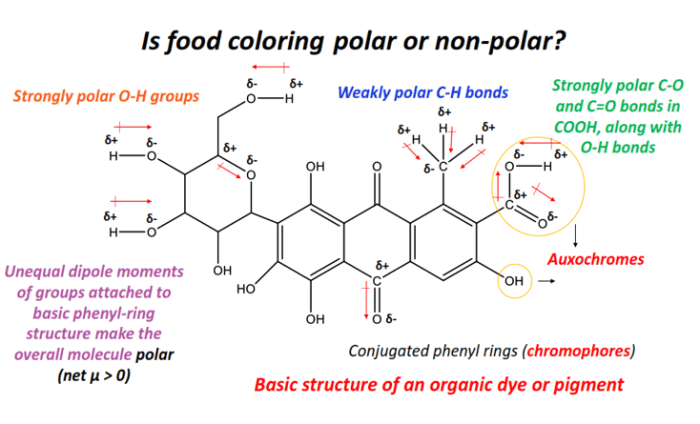
Food coloring, despite its seemingly simple nature, exhibits a complex interplay of intermolecular forces that dictate its solubility and behavior in various solutions. Understanding these forces is crucial to grasping why certain food colorings dissolve readily in water while others might require more effort or specific solvents. The strength and type of these forces are directly linked to the molecular structure of the food coloring itself.The types of intermolecular forces present in food coloring molecules are primarily determined by their chemical structure.
Many food colorings are organic molecules containing polar functional groups, leading to a variety of interactions. These interactions, along with the overall molecular shape, significantly influence the overall polarity and behavior of the molecule.
Types of Intermolecular Forces in Food Coloring
Food coloring molecules typically exhibit a combination of intermolecular forces, including dipole-dipole interactions, London dispersion forces, and in some cases, hydrogen bonding. The relative strength of these forces varies depending on the specific food coloring molecule. For instance, molecules with highly polar functional groups, such as hydroxyl (-OH) or carboxyl (-COOH) groups, will exhibit stronger dipole-dipole interactions and potentially hydrogen bonding.
These stronger interactions lead to higher boiling points and greater solubility in polar solvents like water. Conversely, molecules with fewer polar groups will rely more heavily on weaker London dispersion forces, resulting in lower boiling points and potentially lower solubility in water.
Influence of Intermolecular Forces on Polarity
The presence and strength of intermolecular forces directly impact the overall polarity of a food coloring molecule. Stronger dipole-dipole interactions and hydrogen bonds contribute significantly to the overall polarity, making the molecule more soluble in polar solvents like water. Conversely, a predominance of London dispersion forces, typically found in nonpolar or less polar molecules, results in a less polar character and reduced solubility in water.
This explains why some food colorings dissolve easily in water, while others might require the assistance of a surfactant or a different solvent.
Comparison of Intermolecular Forces and Polarity Across Food Colorings
Different food colorings exhibit varying degrees of polarity due to differences in their molecular structures and the consequent differences in intermolecular forces. For example, a food coloring with numerous hydroxyl groups (like some natural pigments) will exhibit stronger hydrogen bonding and dipole-dipole interactions compared to a synthetic food coloring with primarily nonpolar hydrocarbon chains. The stronger intermolecular forces in the former will result in higher polarity and better solubility in water.
This variation in intermolecular forces directly influences the color intensity and stability of the food coloring in different environments. A highly polar food coloring might be more susceptible to degradation in certain conditions compared to a less polar one.
Impact of Intermolecular Forces on Behavior in Aqueous Solutions
The intermolecular forces between food coloring molecules and water molecules determine the behavior of the food coloring in aqueous solutions. Polar food colorings, with strong dipole-dipole interactions and hydrogen bonding, readily dissolve in water because the water molecules effectively surround and solvate the food coloring molecules. This solvation process overcomes the intermolecular forces within the food coloring, allowing it to disperse uniformly throughout the solution.
Nonpolar food colorings, on the other hand, experience weaker interactions with water molecules and tend to clump together, resulting in poor solubility or the need for emulsifiers to facilitate dispersion. The resulting solution’s stability and color intensity are thus a direct consequence of the balance between the intermolecular forces within the food coloring and between the food coloring and water.
FAQ Insights: Is Food Coloring Polar Or Nonpolar
What determines the color of food coloring?
The color of food coloring is determined by the specific chemical structure of the dye molecule and its ability to absorb and reflect certain wavelengths of light.
Are all food colorings synthetic?
No, some food colorings are derived from natural sources like plants and insects, while others are synthetically produced.
Can the polarity of food coloring affect its safety?
The polarity itself doesn’t directly impact safety, but it influences its stability and interactions with other ingredients, which can indirectly affect safety if degradation or unexpected reactions occur.
How does light affect the stability of food coloring?
Exposure to light can degrade some food colorings, leading to fading or color changes. This is often related to the molecule’s susceptibility to oxidation or photochemical reactions.
